Instrumental Neutron Activation Analysis
a ppm to ppb level multi-element analysis technique
Instrumental Neutron Activation Analysis (INAA) is an analysis technique capable of determining many elements at the same time at very low levels. The technique and its advantages and disadvantages are described here. At the end, a few examples of limits of detection in 3 different kinds of material are given.
Neutron Activation Analysis is a method of qualitative and quantitative element analysis, in which the sample material is irradiated with neutrons in a nuclear reactor. In this process, some stable isotopes are converted into radioactive isotopes. While these isotopes decay, having half-lives varying from seconds to years, they are emitting different kinds of electromagnetic radiation, usually including gamma radiation. This gamma radiation is measured with semi-conductor gamma-ray spectrometers. Specific radionuclides emit gamma radiation of characteristic wavelengths or energies. So when you have a peak in your gamma-spectrum at a certain energy, you can determine with which element it corresponds. The peak area gives information about the amount of this element present in the sample.
Elements that do not yield gamma-ray emitting radionuclides cannot be detected with INAA. The detection limits improves with the amount of radioactivity the element yields and the intensity of the gamma-rays that are emitted. There is no simple rule-of-thumb relating the elements to their detection limits.
Pros and cons
Since INAA hinges on radioactivity production through neutron irradiation, it is the obvious method to use if materials are to be tested or selected for applications in e.g. nuclear power reactors. There, production of long-lived radio-isotopes through neutrons is undesirable because of radiation dose rates for people, as well as of expected decommissioning cost. If we analyze a material with INAA and don’t “see” any long-lived radioactive constituents, it automatically is a good material for such applications. This applies to steel, aluminum alloys as well as concrete and boron-loaded plastics.
The other outstanding feature of the technique is that samples do not need to be dissolved or “torched” for analysis, as in ICP-MS and related techniques. This allows for determination of the halogens (fluorine, chlorine, bromine, iodine) at the mg/kg level in 100 mg samples.
Another important advantage of INAA is that most sample matrices appear to be transparent, because the usual constituting elements - H, C, O, N, P, and Si - hardly form any radionuclides. This makes the method highly sensitive for measuring trace elements, because the main constituents give no signal and do not cause any interferences. Because of this, it is also not necessary to do any sample preparation other than size reduction and (in some cases) drying, making the technique non-destructive. Another advantage is that the technique requires only small amounts of sample material - 100 to 200 milligrams will do.
The way we analyse the spectra in Delft makes it possible to always report an element concentration - when normally a detection limit would be reported, we provide an unbiased concentration with a correct, large relative uncertainty. This results in datasets for larger studies without detection limits or upper limits in them, so that all data have the same meaning and can be interpreted in a straightforward statistical sense.
In INAA, it takes time to complete a full analysis. Because all radioactive isotopes have different half-lives, they can be divided into three categories: short-lived nuclides (half-lives from from a few seconds up to several hours), medium-lived nuclides (a few hours up to several days), and long-lived nuclides (several days up to years). When you want to measure elements which form long lived radionuclides, you need to wait for a few weeks in order for the short- and medium-lived nuclides to decay so that they will not cause any interference, and your detection limits will become better. In the procedure used by the Laboratory for INAA, a complete analysis takes about four weeks. You will find the different protocols and the elements they contain in our price list .
How is an analysis carried out?
The analysis procedure can be divided into four steps: sample preparation, irradiation, measurement, and data processing.

In step 1 sample material, depending on the type of material 50 to 200 mg, is weighed and packed in high purity polyethylene capsules. Because there is almost no sample preparation necessary, contamination is unlikely.
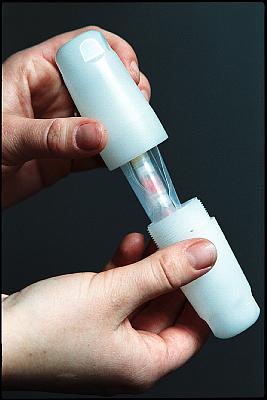
A maximum of 14 capsules filled with sample material, together with a capsule filled with a certified reference material and an empty capsule (the "blank"), are sealed together in polyethylene foil. Comparators are added in order to measure the neutron flux during irradiation.

In step 2, the whole package is packed in an irradiation container, and irradiated by shooting it to a position close to the reactor core by means of a pneumatic irradiation tube system.
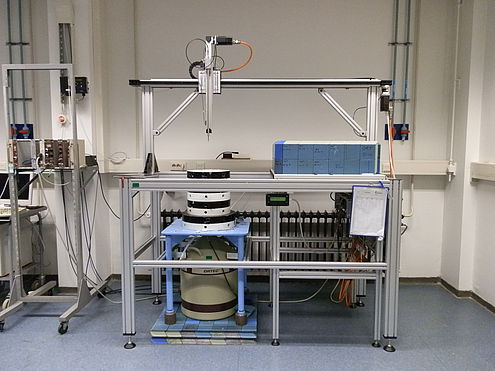
In step 3, the samples are unpacked and measured on either a well-type or a coaxial semi-conductor detector, depending on the amount of radio-activity formed. Depending on which elements you are interested in, a waiting time is applied in order to let the nuclides with shorter half-lives decay, which gives a lower background and less interference. Samples are usually measured for 1 hour, as goes for the reference material and the blank. The comparators are measured for 15 minutes. The results are so-called gamma-ray spectra, where the intensity of the radiation is plotted as a function of photon energy. The spectra exhibit peaks, since each radionuclide emits highly characteristic, well-defined photon energies.
In step 4, the data are processed using a computer system. The peaks are located, the areas and energies determined, and the neutron flux is determined from the comparator spectra. Finally all this information is converted into a list of elements and their concentrations.
In a full analysis, the short-lived nuclides are determined first. Using a fast rabbit system, each sample is irradiated separately (but together with a comparator in order to calculate the neutron flux) for 5 up to 30 seconds at a thermal neutron flux of about 1017 m-2s-1. After a waiting time from seconds (the elements F and Se yield radio-isotopes with half-lives of tens of seconds, and need to be measured as shortly after irradiation as possible) up to 20 minutes, sample and comparator are measured separately.
When the measurements have been completed for all samples, a waiting time of a few days is applied before irradiating them again in order to determine the other elements through longer-lived radio-isotopes. The samples are packed together as described above, and depending on the kind of material, irradiated for 1 to 10 hours at a thermal neutron flux of about 5x1016 m-2s-1. To determine the elements with medium-lived radio-isotopes, all samples are measured 3 to 5 days after irradiation. To determine the long-lived radio-isotopes, the samples are measured again about 3 weeks after irradiation. After this third measurement, all 3 spectra of each sample are processed together.
To give you an idea of the sensitivity of INAA, a few examples of frequently asked elements in different materials are listed below. All limits are based on ± 200 mg of sample material.
Polymer material
| Element | Det. lim. (mg/kg) |
| Element | Det. lim. (mg/kg) |
| As | 0.0056 |
| Ba | 1.8 |
| Br | 0.096 |
| Cd | 0.060 |
| Co | 0.027 |
| Cr | 4.6 |
| Hg | 0.044 |
| Ni | 3.4 |
| Sb | 0.0004 |
| Se | 0.079 |
| Sn | 1.7 |
| Zn | 2.5 |
| Element | Det. lim. (mg/kg) |
| Element | Det. lim. (mg/kg) |
| La | 0.58 |
| Yb | 0.19 |
| Ce | 1.4 |
| Lu | 0.032 |
| Nd | 12 |
| Hf | 0.15 |
| Sm | 0.042 |
| Ta | 0.58 |
| Eu | 0.063 |
| Th | 0.12 |
| Tb | 0.16 |
| U | 0.36 |
| Element
| Det. lim. (mg/kg) |
| Element | Det. lim. (mg/kg) |
Na | 0.5 | Co | 0.05 | ||
K | 30 | Ni | 13 | ||
Ca | 650 | Zn | 1 | ||
Sc | 0.003 | As | 0.07 | ||
Cr | 0.2 | Se | 0.2 | ||
Fe | 25 | Br | 0.3 | ||
Rb | 1 | Zr | 16 | ||
Cd | 1.4 | Sb | 0.05 | ||
Cs | 0.08 | Ba | 12 | ||
La | 0.08 | Ce | 0.1 | ||
Sm | 0.003 | Eu | 0.007 | ||
Yb | 0.03 | Hf | 0.03 | ||
Ta | 0.04 | W | 0.1 | ||
Au | 0.002 | Hg | 0.06 |
| Element | Det. lim. (mg/kg) |
| Element | Det. lim. (mg/kg) |
| Cl | 49 |
| Cu | 10 |
| Al | 1.2 |
| Fe | 41 |
| Mg | 160 |
| Zn | 2.3 |
| Mn | 0.63 |
| Se | 0.32 |
| K | 42 |
| Sr | 22 |
| Na | 0.50 |
| Mo | 1.1 |
Calibration
The system is calibrated using NIST (or equivalent) certified reference materials to relate the observed peak areas, received neutron dose and sample mass to the sample concentrations reported in the end.
If you want more information, please don't hesitate to phone us at ++31 15 2784228, or send e-mail to M. Sarilar. We have a lot more examples with more elements in many different kinds of samples for you on file.