Electronic Structures
The mission of the LM section is to develop new luminescence materials by knowledge based design instead of trial-and-error. To do so we need fundamental knowledge on the level energies of luminescent activator ions and how that all depends on structural and chemical properties of the host compound. In 2012 the chemical shift model was developed by prof. P. Dorenbos that provides a method to establish the vacuum referred electron binding energies (VRBE) in the lanthanide levels. Figure 1 shows that the divalent and trivalent lanthanide ground state level energies change in a characteristic zigzag pattern. With those type of schemes we can predict how deep electrons or holes can be trapped by a lanthanide, and we use them to design or engineer persistent and storage phosphors. The same diagrams are used for understand the preferred valence (2+ or 3+) of the lanthanides in compounds.
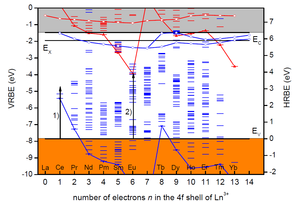
Figure 1. The electronic scheme of electron binding energies in the divalent (red colour) and trivalent (blue colour) lanthanide ground and excited states in LaAlO3 (from Dorenbos J. Phys. Cond. Matt. 25 (2013) 225501)
Combined with spectroscopic data, the chemical shift model model provides the VRBE at the top of the valence band and the bottom of the conduction band in a routine fashion. Today this is the only method that can routinely establish such binding energies at an accuracy level of a few 0.1 eV. This is demonstrated in Figure 2 for the very important class of garnet structure type phosphors applied in modern day light emitting diodes (LEDs).
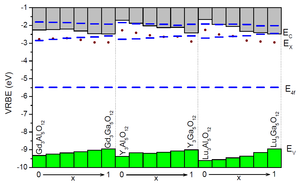
Figure 2. Stacked diagram with VRBE energies of Ce3+ in RE3Al1-xGaxO12 (RE=Gd, Y, Lu) garnet compounds (from Dorenbos, J. Lumin. 134 (2013) 310). The ground and excited states of Ce3+ (blue bars) together with the top of the valence band (green) and bottom of the conduction and (grey) are shown.
Projects in Electronic Structures
6s2-elements
Tl+, Pb2+, and Bi3+ are used as luminescence centre in phosphors and scintillators. Where are the ground state and excited state levels within the bandgap of inorganic compounds? These questions are being solved by combining spectroscopic data with data on valence and conduction and energies from our models.
TM-elements
Like for the 6s2 elements above we aim to arrive at a full understanding on the binding energies in the transition metal ion impurities in compounds.