Catalysis under pressure
The chemical industry is under pressure to develop new processes for the production of plastics and fuels. This requires other catalysts. The Industrial Catalysis Lab is specially designed for work with extreme pressures.
Catalysis is like a magic stone," says Professor Atsushi Urakawa. A catalyst, as we learned in chemistry, is a substance that suddenly makes an unruly chemical reaction possible without itself being converted. That sounds like magic, and that impression is reinforced by the involvement of exotic metals such as platinum.
But 'magic' is no longer good enough. The decline of fossil raw materials has put the chemical industry under pressure to develop new processes for the production of plastics, fuels and fertilisers. New processes require other catalysts that are also less dependent on rare metals. It is Urakawa's mission to significantly shorten the lead time from laboratory to industry. "Given the speed at which the climate is changing, 20-30 years of lead time is no longer an option. We cannot afford to wait that long to make our key chemicals more sustainable." This calls for labs where catalysis is developed under industrial pressure and temperature. "A higher temperature means more aggressive collisions between molecules, and high pressure means a higher chance of hitting them." His hands collide as an illustration. "This is the only way to achieve high conversions."
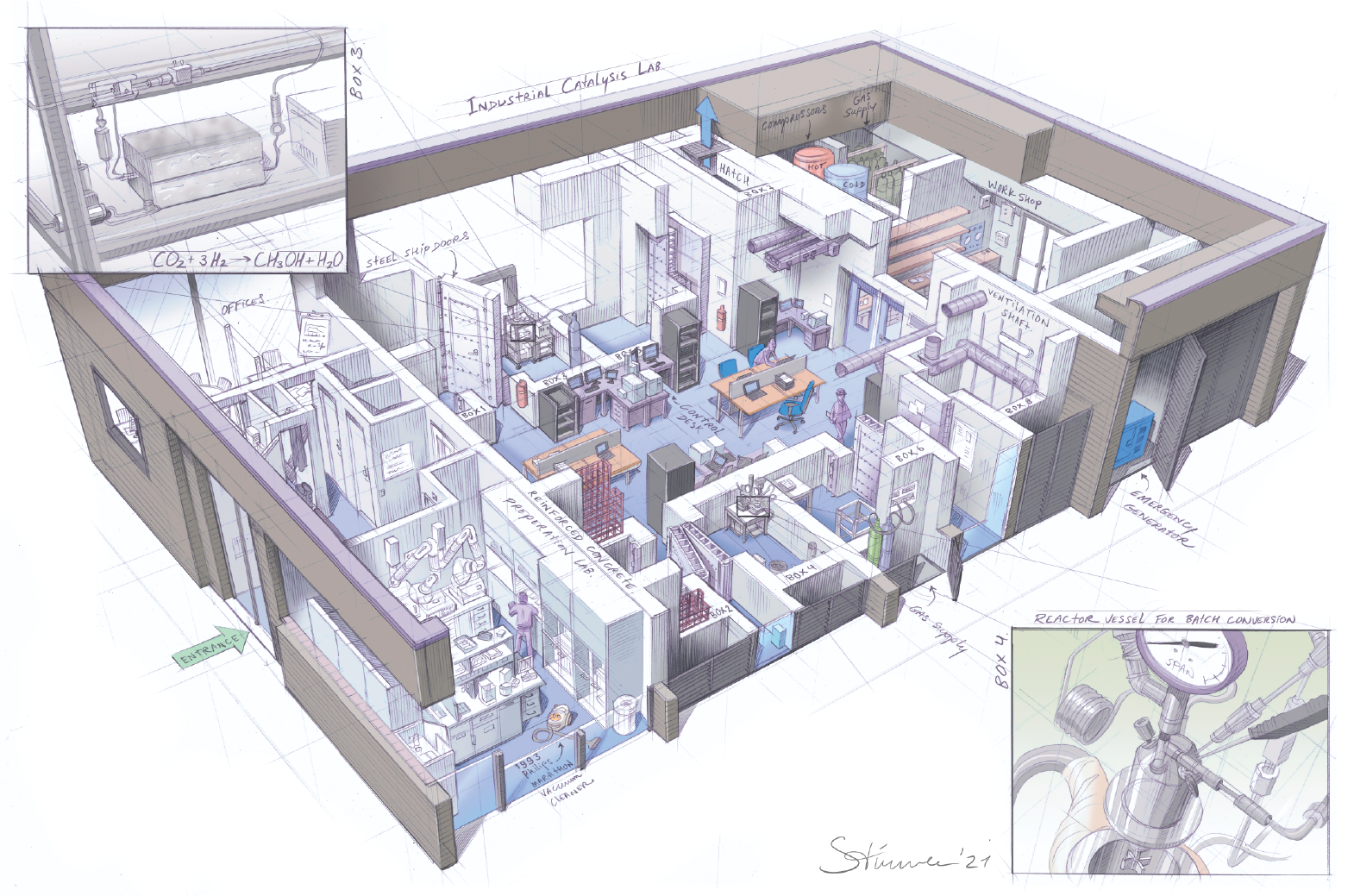
Working pressures of up to 500 bar require a special building. HappelCorelissenVerhoeven architects designed the Industrial Catalysis Lab specially for work with extreme pressures. Experiments take place in one of the eight cells with 50 cm thick walls of extra reinforced concrete, closed by steel ship doors. The rooms are explosion-proof; overpressure is discharged through a special hatch in the roof. The experiment is controlled from outside the cell by a self-designed control system.
In box 3, an experiment is running in which hydrogen and CO₂ are converted to methanol and water. The catalyst is located in a small space between two blocks as big as a brick. Despite the enormous pressure, the tubes are only a few millimetres in diameter.
In box 4, on the other side, a set-up for batch conversion is running, as is usual in the pharmaceutical industry. While the solution is stirred in the reactor, a Raman spectrometer records surface structures and an infrared spectrometer records molecular vibrations. "You can see the chemicals forming on the surface," Urakawa explains.
The 'next generation' catalyst, which is being developed here, is in his conception made up of ordinary metals such as copper, nickel and iron. Composed in alloys or nanostructures and tested at industrial temperatures and pressures. Urakawa is convinced that the 'magic' of the catalyst is ultimately nothing more than the right combination of materials and conditions. Discovering these is the mission of this small black laboratory at the southwest corner of the campus.