Bipolar membranes for intrinsically stable and scalable CO2 electrolysis
The energy transition requires technology to supply sustainable carbon-based chemicals for hard-to-abate sectors such as long-distance transport and plastic manufacturing. These necessary hydrocarbon chemicals and fuels, responsible for 10-20% of the global greenhouse gas emissions, can be produced sustainably by the electrolysis of captured CO2 using renewable electricity.
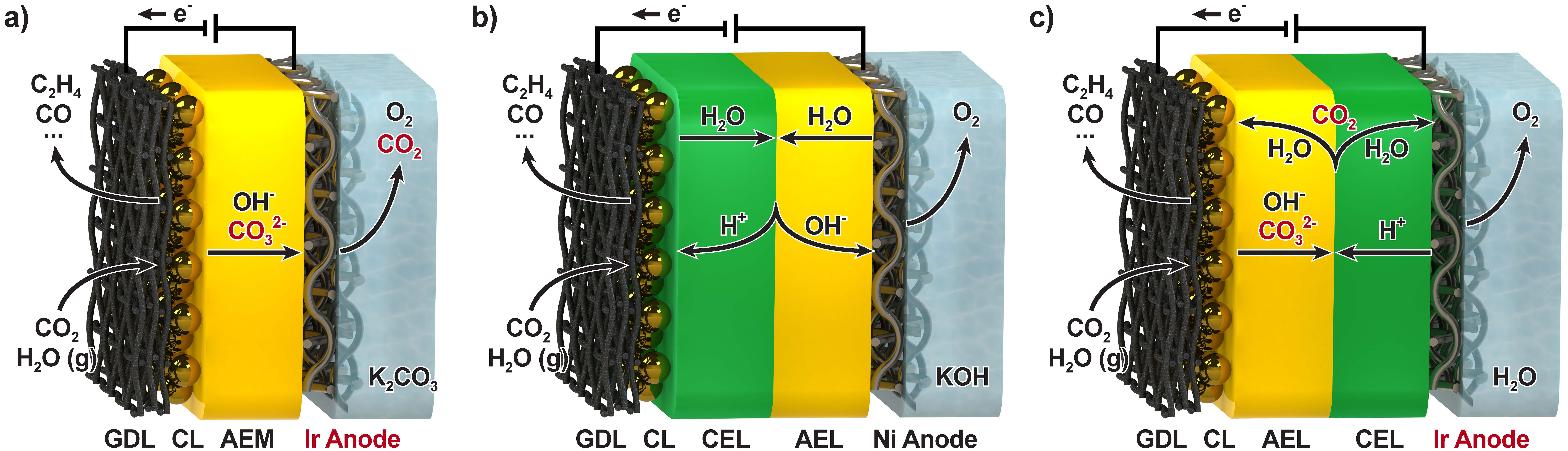
Currently, the state-of-the-art CO2 electrolyzers employ anion exchange membranes (AEMs) to facilitate the transport of hydroxide ions from the cathode to the anode. However, CO2 is crossing the membrane as well, resulting in a loss of reactant and unfavourable anode conditions which necessitates the use of scarce anode materials. Bipolar membranes (BPMs) offer an alternative that addresses the problem of CO2 crossover but still requires research to match the product selectivity of AEM-based systems. Our perspective, a collaboration between groups of David Vermaas, Tom Burdyny and Marc Koper, published in Nature Energy, assesses the potential of BPMs for CO2 electrolysis by looking at CO2 utilization, energy consumption, and strategies to improve the product selectivity.
Abstract
CO2 electrolysis allows the sustainable production of carbon-based fuels and chemicals. However, state-of-the-art CO2 electrolysers employing anion exchange membranes (AEMs) suffer from (bi)carbonate crossover, causing low CO2 utilization and limiting anode choices to those based on precious metals. Here we argue that bipolar membranes (BPMs) could become the primary option for intrinsically stable and efficient CO2 electrolysis without the use of scarce metals. Although both reverse- and forward-bias BPMs can inhibit CO2 crossover, forward-bias BPMs fail to solve the rare-earth metals requirement at the anode. Unfortunately, reverse-bias BPM systems presently exhibit comparatively lower Faradaic efficiencies and higher cell voltages than AEM-based systems. We argue that these performance challenges can be overcome by focusing research on optimizing the catalyst, reaction microenvironment and alkali cation availability. Furthermore, BPMs can be improved by using thinner layers and a suitable water dissociation catalyst, thus alleviating core remaining challenges in CO2 electrolysis to bring this technology to the industrial scale.